In everyday clinical practice, the therapeutic pathway of autologous CAR T-cell starts from leukapheresis, but the drug is infused on average 4-7 weeks later. Between leukapheresis and CAR T infusion patients may die following disease progression or exit the therapeutic pathway due to comorbidities or other reasons, thereby introducing a potential bias in the estimation of survival benefit gained with the drug. Moreover, substantial differences may occur (e.g., timing of leukapheresis and enrollment, use of bridging chemotherapy, lymphodepleting regimens) between trials' designs and every-day clinical practice. All of this precludes a robust comparison among the different available CAR T products and the therapies which share the same indication. Considering analyses limited to treated patients with endpoints estimated from date of infusion should be avoided to prevent misleading treatment choices.
We conceived a project involving researchers and cooperative groups worldwide which already published real world effectiveness data on per-protocol (PP, i.e. from infusion) or on intention-to-treat (ITT, i.e. from leukapheresis taking into account also subjects who were scheduled for but finally did not receive CAR T) populations with the aim to re-estimate efficacy endpoints in both modalities. This would offer the clinicians the possibility to draw more meaningful interpretations of the data according to these two different approaches (ALMA IDEA 2022 CUP:J33C22001420001).
First a systematic review was conducted searching for real world studies on the effectiveness of anti-CD19 CAR T-cell products administered to large B-cell lymphoma patients in third line and beyond between 2017 (after FDA approval) and October 2022. For each study, we asked researchers about both infused and not infused patients, for overall response rate (ORR), complete response rate (CRR), median follow up, median survivals (event-free [EFS], progression-free [PFS] and overall [OS]) duration of response (DoR). ITT EFS, the new endpoint added for our project, was defined as the time the patients remained in the CAR T pathway from leukapheresis to exit due to any cause. When applicable, meta-regressions, metanalyses and pooled analyses were also performed.
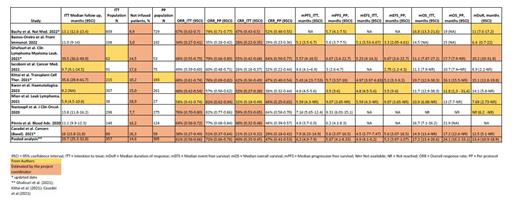
The results overview with the 10 selected studies is reported in Table 1 (Bachy et al. Nat Med. 2022; Bastos-Oreiro et al. Front Immunol. 2022; Ghafouri et al. Clin Lymphoma Myeloma Leuk. 2021; Iacoboni et al. Cancer Med. 2021; Kittai et al. Transplant Cell Ther. 2021; Kwon et al. Haematologica. 2023; Mian et al. Leuk Lymphoma. 2021; Nastoupil et al. J Clin Oncol. 2020; Pinnix et al. Blood Adv. 2020; Casadei et al. Cancers (Basel). 2021). Rates of not infused patients ranged from 3% to 29%. Meta-regressions did not indicate a statistically significant influence of the different ratio of infused/not infused patients among studies, nor the different lengths of the median follow up seem to bias project results. Metanalyses for ITT ORR and CRR resulted in 57% and 38% respectively while the PP ORR and CRR were 68% and 45% respectively. Although centers kept track of all patients scheduled for CAR T, around 50% did not record the exit date (decision of not infuse, due to any cause) for not-infused subjects, reflecting the inability to calculate the ITT EFS. Three studies reported a median PP PFS shorter than the ITT PFS, which may indicate a high mean time from leukapheresis to infusion, worthy of further investigation. For all studies but one median PP OS was higher than ITT OS: OS may be over-estimated when using a PP analysis.
We should be using an ITT analysis, as reporting data in which we do not include the patients who underwent leukapheresis and were never treated could introduce a significant bias in results interpretation.
Hematologists should understand which is the most clinical meaningful endpoint for this new therapeutic approach to reach effective results: have we to consider CAR T-cell therapy as a drug or a therapeutic pathway?
The importance of considering this new treatment option as a pathway should translate into implementation of CAR T patient registries that include ITT as an endpoint to establish population-level effectiveness of these drugs. In addition, considering both PP and ITT analyses as well as infused and not infused patients provides a complete overview and leads to more information.
Disclosures
Zinzani:ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees. Casadei:Takeda: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; Roche: Speakers Bureau; Lilly: Speakers Bureau; Novartis: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Locke:ASH: Other: Travel Support; Emerging Therapy Solutions: Consultancy, Other; BioPharma Communications CARE Education: Other: Institutional; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Travel Support; Leukemia and Lymphoma Society: Other; Cowen: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Cellular Medicine Group: Consultancy; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Daiichi Sankyo: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees; EcoR1: Consultancy; National Cancer Institute: Other; Society for Immunotherapy of Cancer: Other; Clinical Care Options Oncology: Other; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; GammaDelta Therapeutics: Consultancy; Imedex: Other; Caribou: Consultancy; Calibr: Consultancy; CERo Therapeutics: Other: (Institutional); Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gerson Lehrman Group (GLG): Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Jain:Loxo@Lilly: Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Myeloid Therapeutics: Consultancy, Honoraria; Incyte: Research Funding. Voorhees:Novartis: Consultancy; Morphosys: Research Funding; Incyte: Research Funding; AstraZeneca: Research Funding; Recordati: Consultancy, Research Funding. Kittai:Eli Lilly: Consultancy; BeiGene: Consultancy, Research Funding, Speakers Bureau; Abbive: Consultancy; AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy; KITE: Consultancy; BMS: Consultancy. Bastos-Oreiro:Incyte, Kite: Consultancy; BMS, Kite, Novartis, F. Hoffmann-La Roche Ltd, Incyte, Abbvie: Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd, Kite, SEHH, AMHH: Research Funding; Gregorio Maranon Hospital: Current Employment, Membership on an entity's Board of Directors or advisory committees; SEHH, AMHH: Membership on an entity's Board of Directors or advisory committees. Martin Garcia-Sancho:Kyowa Kirin: Consultancy, Honoraria; Clinigen: Consultancy; Eusa Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead / Kite: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; ADC Therapeutics America: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Ideogen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd, BMS / Celgene, Kyowa Kirin, Novartis, Gilead / Kite, Incyte, Lilly, ADC Therapeutics America, Miltenyi, Ideogen, Abbvie, Sobi: Consultancy; F. Hoffmann-La Roche Ltd, BMS/Celgene, Janssen, Gilead/Kite, Takeda, Eusa Pharma, Abbvie: Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Terol:Hematologist, Head of the lymphoma Unit, Department of Hematology, Insitute of Research INCLIVA, University of Valencia, Spain: Current Employment; Beigene, Gilead, F. Hoffmann-La Roche Ltd, Abbvie, Janssen: Consultancy; Gilead: Research Funding; F. Hoffmann-La Roche Ltd, Janssen, Gilead, Takeda, Abbvie, Beigene: Speakers Bureau; F. Hoffmann-La Roche Ltd, Janssen, Gilead, Takeda, Abbvie, Beigene: Membership on an entity's Board of Directors or advisory committees. Iacoboni:Abbvie: Honoraria; Miltenyi: Consultancy, Honoraria; Autolus: Consultancy; Novartis: Consultancy, Honoraria; Janssen: Honoraria; MSD: Honoraria; Gilead Sciences: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; AstraZeneca: Honoraria. Barba:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kwon:Jazz: Speakers Bureau; Pfizer: Speakers Bureau; Kite-Gilead: Consultancy, Speakers Bureau. Reguera:AMGEN: Speakers Bureau; KITE: Speakers Bureau; BMS: Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Hill:Genentech: Consultancy, Other: Advisory board, Research Funding; Bristol Myers Squibb: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Incyte: Consultancy; Gilead: Other: Advisory board; AbbVie: Consultancy, Other: Advisory board, Research Funding. Bachy:Incyte: Honoraria; Pfizer: Honoraria, Other: Personal Fees; Takeda: Honoraria; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Novartis: Honoraria, Other: Personal Fees; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Roche: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees. Morschhauser:F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy; F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria. Thieblemont:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment; Bayer: Honoraria; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Hospira: Research Funding; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Janssen: Honoraria, Other: Travel Expenses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal